
Supporting Research
Impact of Antimicrobial Stewardship program interventions on costs.
Evidence for Antimicrobial Stewardship was found at: Centers for Disease Control and Prevention
Results for Return on Investment
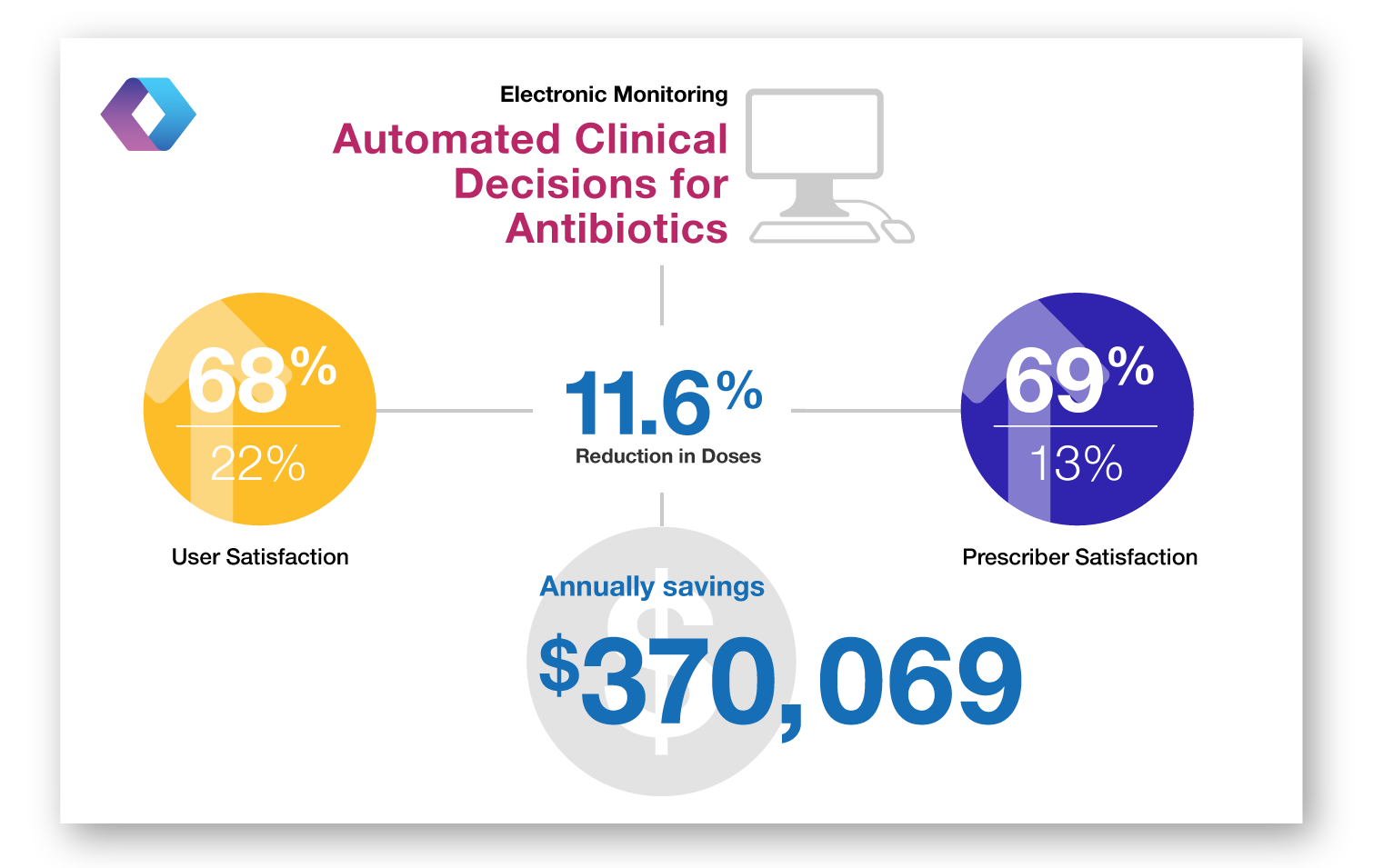
Electronic Monitoring – Implementation of a web-based program to provide automated clinical decision support, faciliate approval and real-time communication with prescribers related to antibiotics.
Restriction – Eight IV antibiotics (amikacin, aztreonam, ceftazidime, ciprofloxacin, fluconazole, imipenem-cilastatin, ofloxacin, and ticarcillin-clavulanate, (aztreonam was added to list two months into intervention) previously needed approval, but enforcement of restrictions lax. All staff informed that ID approval needed for dispensement and that pharmacists needed to be informed in writing or by telephone. Enhanced enforcement included issuing warnings to pharmacists if restricted antibiotics dispensed without ID approval.
Electronic Monitoring – randomized controlled trial of active monitoring by antimicrobial management team of all restricted antibiotic orders with a computer system compared to a group without the computer system to alert for inadequate antimicrobial therapy.
Conversion – CAP pts randomized to conventional course of IV antibiotics vs. abbreviated course of IV antibiotics followed by conversion to oral antibiotics conversion-CAP pts randomized to conventional course of IV antibiotics vs. abbreviated course of IV antibiotics followed by conversion to oral antibiotics.
Conversion – Pharmacists contacted physicians to recommend oral antibiotics conversions from selected broad-spectrum and high-cost parenteral antibiotics for patients with predetermined mild to moderate clinical conditions.
Multidisciplinary – Implementation of new restrictions in antibiotic formulary for broad-spectrum antibiotics and restrictions for antibiotics maximum daily dosage.
Order form – Creation of an order form with preset dosing orders for clindamycin, cefazolin and metronidazole. Physicians still had option to choose own dosing schedule on the form.
Restriction-creation of formulary restrictions for vancomycin and select aminoglycocides and cephalosporins, which would require obtaining ID approval for use. ID told not to reject requests but to use persuasion.
Restriction-All physicians wanting to prescribe cephalexin had to seek approval from ID physician.
Guidelines – Establishment of guidelines that cefazolin is cephalosporin of choice. Physicians contacted if orders deemed to be noncompliant.










Guidelines – Established restrictions on use of 2nd generation cephalosporins and aminoglycosides requiring obtaining pharmacy approval before drug disbursement